| Product Specification |
THE TOXIN™ | Future R&D |
|---|---|---|
| Indication | Glabella line | |
| Strain | ATCC3502 (NCTC 13319) |
Type B Type E PFS |
| Origin of Strain | Officially acquired from EU | Officially acquired from EU |
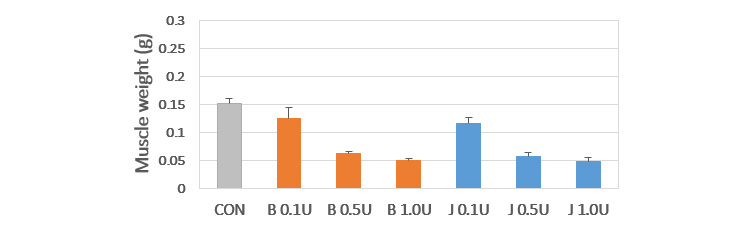
| Type | Type A | Type B Type E |
| Model | 50U, 100U, 200U | on developing |
| Duration | 3~4M for glabella line | |
| Indication |
Facial wrinkle Masseter muscle reduction Spasticity Spasm, Pain Hair loss, Scar |
|
| On set time | 2-3 days | 1 day |
| Conversion ration with Botox, Allergan |
1:1 | on developing |
| Purity | 98.9% ≤ | on developing |
| Total protein | 2.4 ng / 100 U | on developing |